Arctic ice is frozen seawater, so why is it fresh instead of salty?
Good question! The sea ice that covers most of the Arctic Ocean in winter and some of it in summer is seawater that has frozen because of the low temperatures. Given that seawater is salty, you might expect frozen seawater to be salty as well. But it’s not.
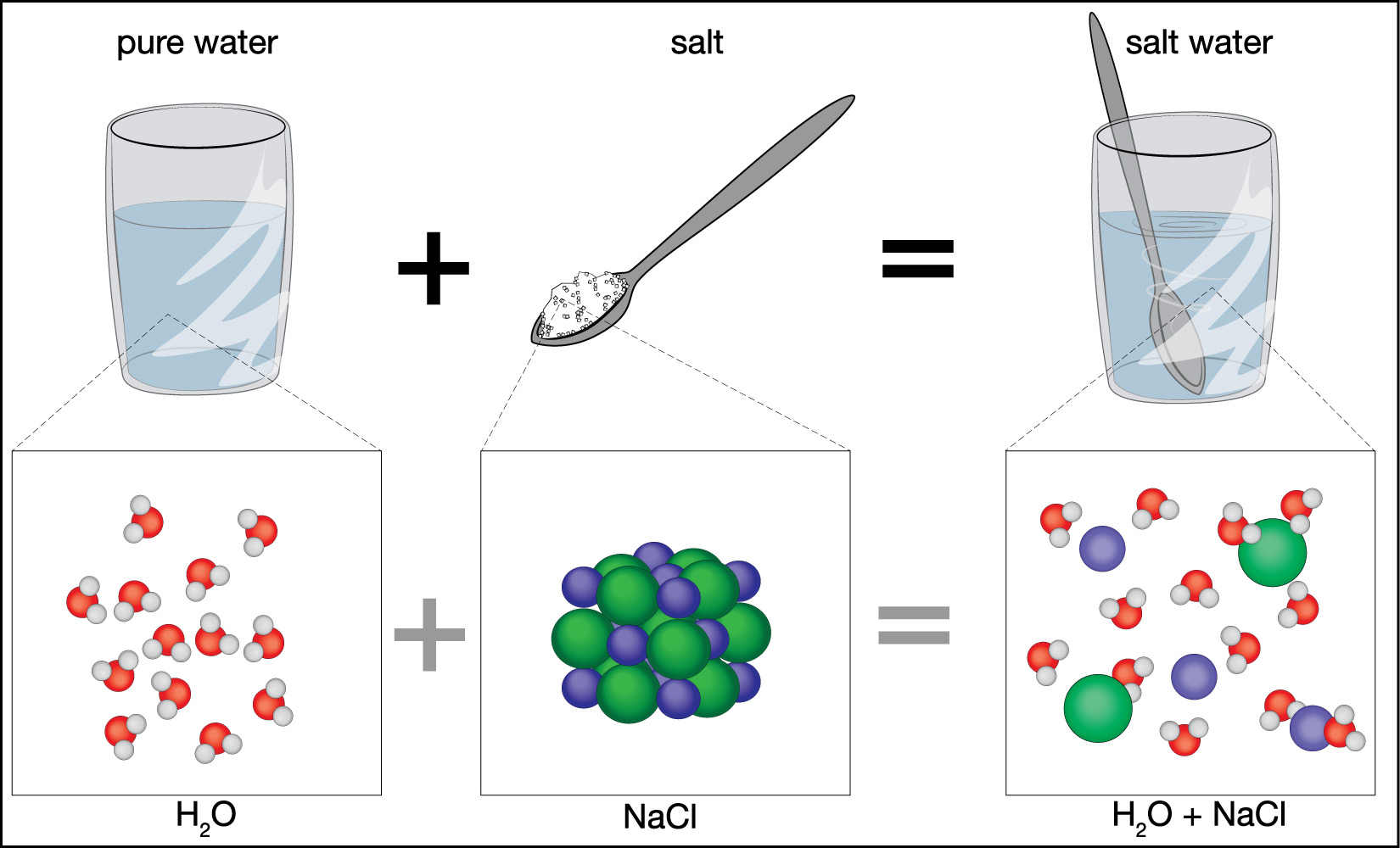
To understand why, look back at Figure 6.1.2–2. Notice that the salt atoms that are dissolved in salt water are larger than the water molecules themselves. As a result, when the water molecules freeze to make a crystal structure, there is simply no room for the salt atoms, so they get pushed out of the ice as the seawater freezes.
This fact has important implications to the salinity of the Arctic ocean. Because freezing forces out salts, the ocean water that remains liquid (under the ice) becomes saltier in the winters as ice freezes. Then, as ice melts in the summers, the salinity decreases. (You can see this annual cycle if you re-watch Video 6.1.2–5.)
This also has important implications for climate change. As we’ll discuss in Section 7.2.1, global warming is leading to reduced ice coverage in the Arctic Ocean, and this means that the ocean salinity is decreasing in this region as a result. The lower salinity, in turn, can affect ocean currents, fishery production, and more. All of these changes are likely to have detrimental effects on us humans, which is just one more reason why so scientists are so concerned about climate change.